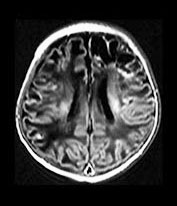
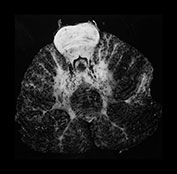
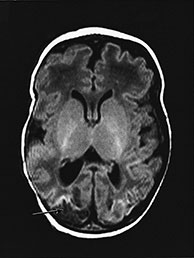
Part 4 – Chapter 10 – Neonatal brain infection
Neonatal brain infection
Susan Blaser, Venita Jay, Laurence E Becker and E Lee Ford-Jones
10
Chapter Contents
Neonatal CNS infections, whether acquired in utero (congenital), intrapartum or postnatally remain an important cause of acute and long-term neurological morbidity. Pathologic features and associated imaging patterns depend upon the stage of development of the CNS, the affinity of a specific infective agent for a specific CNS cell type, and the ability of the host to respond to that insult. With the discovery of cytokines and adhesion molecules, and the demonstration of lymphocyte recruitment across the blood–brain barrier (BBB), the CNS is now known to be able to mount and propagate an inflammatory response to infections. This immune response has been invoked in neonatal brain damage even when the maternal infection, which may have had its onset before pregnancy, does not directly involve the fetal brain. The effects of infection on the rapidly developing brain, which continually evolves in its susceptibility to damage, in association with an evolving immune system lead to complex patterns of pathology and imaging features dissimilar to those seen in an older child ill with a similar infectious agent.
Certain factors aid in the diagnosis and differentiation of the congenital and neonatal infections. The etiologic agent may be known if the mother was exposed to an infectious agent or had a symptomatic infection. Conclusive diagnosis is dependent upon comprehensive clinical evaluation, ophthalmologic examination, microbiological testing of the infant and mother, and serial follow-up serology of the infant. Additionally, the actual clinical presentations of infection in the neonate are different for viruses, bacteria and parasites. Infants with bacterial infections are likely to present with sepsis, while those with cytomegalovirus (CMV) or toxoplasmosis infections may be clinically asymptomatic at birth despite their obvious intracranial involvement on imaging or ophthalmologic examination. Neonates with viral infections may present with active hepatitis, skin vesicles or petechiae.
The mechanism of infection and damage is also different amongst the infectious agents, leading to more specific imaging and pathologic appearances. Viruses, for example, tend to produce a selective necrosis of specific cell types, whereas bacteria and fungi are less selective. Also, different patterns of calcifications on CT or pathologic specimens are typical for the various STORCH (syphilis, toxoplasmosis, rubella, CMV, human immunodeficiency virus (HIV) and herpes simplex) infections, and the timing of insult during fetal life may lead to either teratogenic or encephaloclastic effects. Clinical and imaging differentiation amongst these disorders and amongst their respective infective agents is, therefore, frequently possible3, 8, 20, 21, 73, 83.
< prev |
top |
contents |
next >
MRI should include conventional T1 and T2 weighted imaging in at least two planes in any infant with suspected infection. The sagittal plane is useful for detecting thrombosis in the sinuses. Axial images should always include full posterior fossa views to visualize the transverse sinuses. The use of contrast is mandatory for detecting early changes within the meninges or parenchyma and for establishing the full extent of any abnormalities. The role of diffusion weighted imaging in patients with infection is now being recognized. Time allowing, a fluid attenuated inversion recovery (FLAIR) sequence may give additional postcontrast information.
< prev |
top |
contents |
next >

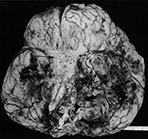
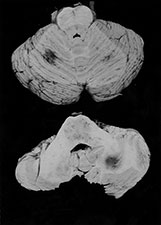
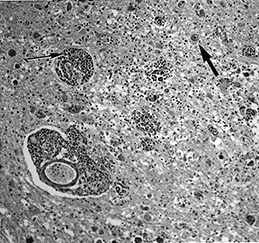
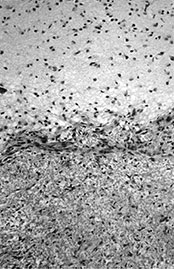
Fig. 10.1 Tubercular infection with vasculitis. Several vessels are shown in this field with transmural inflammatory infiltrate (arrow). Arteritis (arrowhead) is a common complication in tubercular meningitis. Vasculitis of arteries and veins may lead to thrombosis and hemorrhagic infarction. (H&E stain, low power view.)
Predisposing factors for bacterial infections in the newborn include maternal sepsis or chorioamnionitis, maternal cervical colonization of agents such as group B streptococcus, prolonged rupture of membranes prior to delivery, complications of labor and delivery, and deficiencies of cell-mediated or humoral immunity in the infant. Nosocomial and iatrogenic etiologies include exposure to reservoirs of pathogens within the neonatal intensive care unit, and invasive procedures such as endotracheal intubation, central vascular access, or CSF diversion. Group B streptococcus and Escherichia coli (E. coli) are the most common bacteria causing significant disease in the neonate. Infection from enteric organisms is more frequent during the first 2 weeks of life particularly with the use of intrapartum group B streptococcus prophylaxis, while those from streptococcus and staphylococcus species become more prevalent during the second 2 weeks of life. There is a particular propensity for E. coli to infect the neonate as the 19-S macroglobulin fraction which contains maternal antibodies to coliform bacteria does not cross the placenta, leading to a lack of passively acquired immunity to Gram-negative organisms. Other important agents in the neonatal period are Listeria monocytogenes and other members of the Enterobacteriaceae group, Citrobacter sp. and Enterobacter sp. Staphylococcus aureus and Epidermidis infections are particularly common in surgical neonates and those with indwelling shunts or central venous lines. By 2 months of age, with loss of passive immunity, there is a rise in CNS infections from Hemophilus, Pneumococcus and Meningococcus.
Hematogenous spread of bacteria from omphalitis, urinary tract infection or pneumonia leads to co-existence of peritonitis, arthritis, and in some cases, meningitis. Entry into the CNS requires the bacteria to cross an epithelial– mucosal barrier, such as upper respiratory tract, intestinal mucosa, or umbilical stump, to reach the blood stream. The actual site and mechanism of egress of bacteria from the blood stream into the CSF is not fully defined in every case. Pathways include extension through structures without intact BBB such as the choroid plexus, factors released by the organism which allow intracellular transport and direct vessel wall invasion, and vascular compromise with direct invasion of adjacent necrotic brain tissue. Non-hematogenous pathways leading to CSF infection include direct extension, for example from an overlying scalp infection, or direct inoculation via ventricular shunt or puncture.
Neonates with bacterial CNS infections frequently present with apnea, lethargy and other signs of fulminant systemic illness or shock rather than signs of meningeal irritation (Fig. 10.2). Bulging fontanel and seizures are non-specific features seen in association with any cause of increased intracranial pressure in this age group, even when there is no intracranial involvement by the infecting agent. Premature infants, due to the deficiencies in neonatal host defense mechanisms and to higher permeability of leptomeninges, are even more susceptible than full-term neonates to bacterial infections, including meningitis. They also have a much higher mortality. Mortality rates for infants with group B streptococcus, for example, range from 70% for prematures weighing under 1 kg to 10% or less in infants weighing over 2.5 kg4, 40, 48, 57, 70, 85.
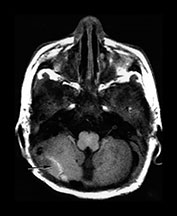
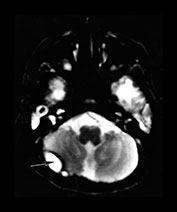
Complications of bacterial meningitis are extremely common in infants under 6 months of age. Follow-up imaging is therefore suggested in neonates to exclude the presence of complications requiring surgical intervention or change in therapy. These complications include cerebritis, infarction (Fig. 10.1), brain abscess, subdural effusion or empyema, sinus thrombosis, ventriculitis and hydrocephalus. In uncomplicated bacterial meningitis, organisms and purulent enhancing inflammatory cell exudate fill the sulci over the hemispheres and extend along the perivascular spaces. Engorgement of surface vessels occurs and leaky blood–meningeal barrier from perivascular inflammation leads to meningeal and cisternal enhancement. The presence and extent of this enhancement is more consistently documented on MR than CT, but can be demonstrated on both imaging modalities (Fig. 10.2 and Fig. 10.3). Initially, the exudate consists of granulocytes, later monocytes increase and there is progressive organization of the exudate with fibroblast proliferation. Subdural effusions, empyemas, leptomeningeal scarring and fibrosis, and arachnoiditis with loculated CSF further complicate neonatal meningitis, although these collections have been reported to be less common in neonates than in infants older than 2 months of age. Sterile subdural effusions are present in up to half of children presenting with meningitis within the first year of life. A very small percentage of these, approximately 2%, will develop into empyemas. Subdural effusions and empyemas may require surgical sampling for differentiation, although useful imaging features suggesting empyema formation are enhancing rinds and signal intensity which fails to match CSF. Empyemas are more likely to contain proteinaceous fluid, with signal intensity on T2W images greater than CSF. Subjacent brain signal is also more likely to be abnormal in the presence of empyemas than in the presence of an effusion. Brain signal subjacent to empyema or infected collections is variable, with increased signal on T2W imaging reflecting edema or ischemia25, 34, 82, 85.
Decreased signal intensity on T2W imaging of the adjacent cortex and subcortical white matter likely reflects the increased vascular perfusion and loss of vessel autoregulation in the underlying brain (Fig. 10.4).
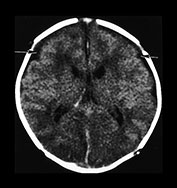
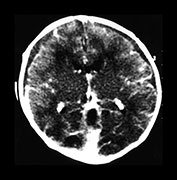
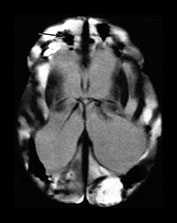
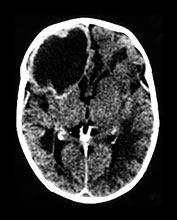
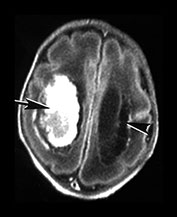
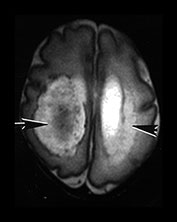
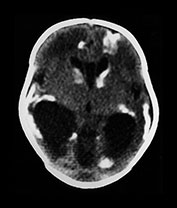
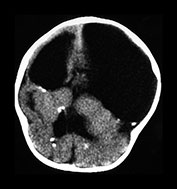
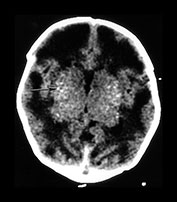
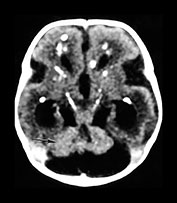
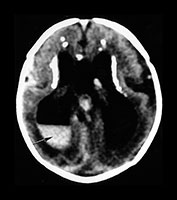
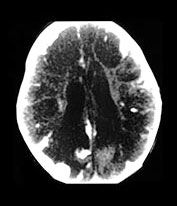
Fig. 10.2 Group B streptococcus with watershed infarction. Unenhanced (a) and enhanced (b) axial CT reveals poor occipital gray–white differentiation, cortical enhancement, and the presence of bilateral empyemas (arrows) in a 7-day-old full-term infant who presented in shock with seizures and fixed pupils.
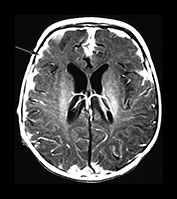
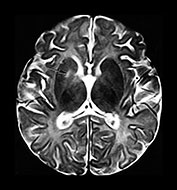
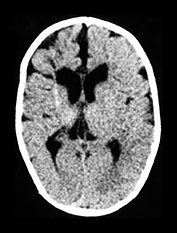
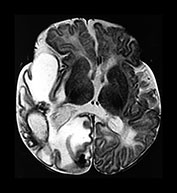
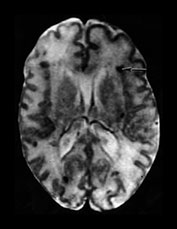
Fig. 10.3 E. coli meningitis with lenticulostriate infarction. FLAIR (a) axial image in a full-term neonate shows increased signal filling the sulci (arrow). This inflammatory exudate enhances (arrow) on T1W (500/20) axial image following administration of gadolinium-DTPA (b). Focal increased signal of infarction (arrow) is present in the right basal ganglia on T2W (3000/120) image (c). Thick basal purulent exudate (arrow) obscuring vessels is seen on autopsy specimen (d) in another infant who died from E. coli meningitis.
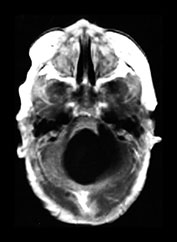
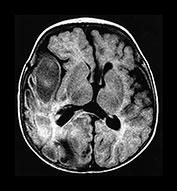
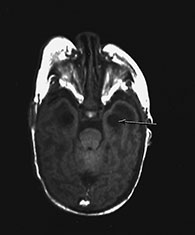
Fig. 10.4 E. Coli empyema T1 (600/14) (a) and T2 weighted (3000/120) (b) axial images in a 3-week-old with complex congenital heart disease and E. coli meningitis demonstrate a focal epidural collection effacing the right cerebellar hemisphere (arrow). Note the subtle low signal on T2W image of the surrounding brain tissue, likely due to local vascular congestion. This collection was unsuspected before imaging and was surgically proven to be an empyema.
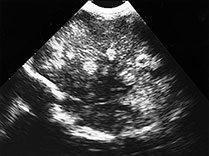
Ventricular enlargement may result from occlusion of the foramina of Monro, the aqueduct of Sylvius, and the 4th ventricular outlets by fibrinous inflammatory exudate which occurs even in the absence of ventriculitis. Ventriculitis, however, is a common and early component of neonatal meningitis, as the choroid plexus is a common site of bacterial entry into the CNS. Intraventricular exudate covers the choroid plexus, disrupts the ependymal lining and leads to subependymal venous thrombosis and eventual subependymal necrosis. Imaging features of ventriculitis should be sought in all patients with meningitis, but may be subtle in early cases. Pus-fluid levels and ependymal thickening may be seen on all imaging modalities, including sonography. A periventricular band of increased echogenicity has been reported to be a suggestive feature on brain sonography. This band may also be seen with chemical ventriculitis following intraventricular hemorrhage, in association with interstitial edema related to obstructive hydrocephalus, or with periventricular calcifications in STORCH infections. Ependymal enhancement and thickening on MR and CT may also be seen following intraventricular hemorrhage. Intraventricular cyst formation and bridging ependymal strands lead to ventricular isolation and difficult to treat loculations in survivors in the convalescent and chronic stages
8, 22, 34, 63, 84 (Fig. 10.5).
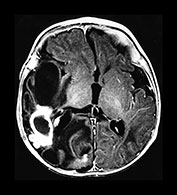
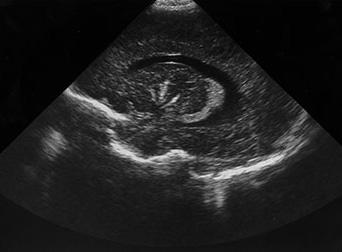
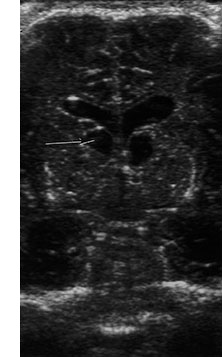
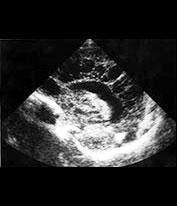
Fig. 10.5 Pseudomonas with ventriculitis. Parasagittal head sonogram (a) performed at 15 days of age in the evaluation of systemic Pseudomonas sepsis, dropping hemoglobin, and low platelet count in a 31 week gestational age twin demonstrates multiple echogenic foci within brain substance. T2 (3000/120) (b), and unenhanced and enhanced T1W (600/22) axial images (c,d,e) from an MR performed at 30 days of life show loculated, isolated ventricles, multifocal brain hemorrhage (arrow), and adherent choroid plexus glomus. Spinal empyemas were also identified (f). Subsequent MR at 2 years of age (not shown) confirmed extensive periventricular brain substance destruction and calcification in a shunted and severely developmentally delayed child.
Brain tissue involvement, or cerebritis, follows extension of exudate along the perivascular spaces. Cerebritis is commonly present at autopsy in cases of meningitis; however, the features of gyriform signal change and enhancement are not always present during the early stages of brain involvement. These foci of signal change and enhancement may resolve with therapy, or progress. As the infection progresses, exudate covering the surface veins leads to thrombophlebitis and cortical venous occlusion in adults. Venous occlusions in neonatal meningitis are usually secondary to fibrinous thrombi within the congested veins, rather than extension from the major venous sinuses and occur earlier in the course of disease in infants than they do in adults (Fig. 10.6). Meningitis-related infarctions are usually venous, although occasionally there is arterial infarction from basal leptomeningeal arterial involvement (Fig. 10.1 and Fig. 10.3). Infarcts from either route occur in approximately 30% of neonates with meningitis and are frequently large and hemorrhagic. Cortical and white matter infarctions occur, and white matter involvement is significantly more common in the neonate than in the adult. Subependymal and periventricular venous occlusion and infarction contribute to the damage. Residual gliosis, encephalomalacia and porencephaly are present on follow-up imaging
15, 25, 34.
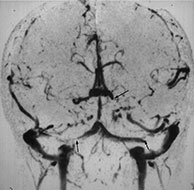
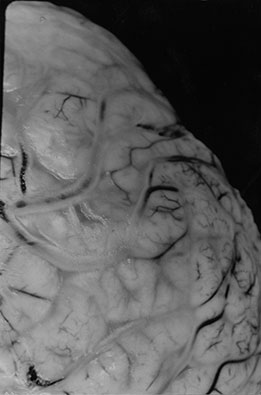
Fig. 10.6 Sinovenous thrombosis in a neonate presenting with sepsis. Unenhanced sagittal (a) and axial (b) T1W and T2W (c) and 2D TOF MR venography (d) views demonstrate superior sagittal sinus (a) (arrow), left internal cerebral and medial atrial venous thrombosis and associated hemorrhagic frontal white matter and basal ganglia infarction. The venogram (d) shows no filling of the left internal cerebral vein (top arrow) and poor filling of the tranverse sinuses (bottom arrows). Close up of meningeal surface in a case of acute bacterial meningitis. Note purulent exudate filling the sulci and covering the superficial veins in another patient, some of which appear to be thrombosed (e).
Brain abscesses may result from a hematogenous source, from direct inoculation, or by local spread particularly into infarcted brain. Unlike the adult, most brain abscesses in the newborn result as a complication of bacterial meningitis. Five to 10% of neonates with meningitis will go on to develop abscesses, with the exception of those infected with Citrobacter sp. in whom 75% will develop abscesses. When the organisms are of low virulence, early and late stages of cerebritis may be seen, with minimal BBB disruption and contrast enhancement. In the older infant, rapid development of enhancement occurs, related to the acute inflammatory reaction. Enhancement may be diffuse prior to the development of a collagen capsule and a necrotic center. Imaging features of well-developed abscesses in the older infant include peripheral edema and central necrosis with abscess fluid hyperintense to CSF on short TR (repetition time) scans and hyperintense to gray matter on long TR scans. Concentric zones of varying intensity are common, as are abscess capsules with signal hyperintense relative to brain on short TR and hypointense relative to white matter on long TR scans. The capsular signal intensity is likely due to the presence of free radicals from macrophages. Immunocompromised patients and neonates, whose immune system is immature, often fail to mount a significant inflammatory response and subsequently have misleading or poorly developed imaging findings. Neonatal abscesses are often large and lack well-defined capsular formation. Mortality is significantly higher in the youngest infants29, 45, 85(Fig. 10.7 and Fig. 10.8) although focal frontal cerebral abscesses may be clinically silent in the preterm infant and the long-term outcome surprisingly good (Fig. 10.8).
Fig. 10.7 Citrobacter – serial CT imaging in a full-term infant developing sepsis at day 8 Citrobacter meningitis with sterile ‘abscess’, due to brain infarction. Initially cerebritis with frontal lobar swelling is seen at 11 days (a). Large areas of white matter necrosis with rim enhancement (b) are subsequently seen at 19 days. Proven abscess (c) eventually shows mass effect and ëroundingí is shown at 28 days. Follow-up CT (d) at 4 months of age at presentation in status epilepticus demonstrates atrophy, focal scarring, and faint calcification.
SPECIFIC ORGANISMS
Group B streptococcus (GBS)
GBS is a partially preventable, but unfortunately common etiologic agent of neonatal meningitis. Systemic group B streptococcal infections occur in up to 10% of neonates in whom there is maternal colonization of the cervix, leading to a rate of 1–5 per 1000 live births. Meningitis co-exists in approximately 5–10% of those. Myelitis has been reported. Prematurity, prolonged rupture of membranes, and maternal chorioamnionitis are known to increase the risk of neonatal infection. Infections may appear within the first 24 h, or up to 3 months of age. Neonates are more likely than the older infants to have a shock-like presentation and fulminant course, and very low birth weight infants have a mortality approximating 70%. Those with later-onset disease have a more insidious presentation with meningitis and osteomyelitis. Necrologic sequelae in survivors of GBS meningitis include hydrocephalus, developmental delay and seizures (Fig. 10.2)4, 32, 69.
Listeria monocytogenes
Listeria monocytogenes, a Gram-positive bacteria, has a tendency to infect the very young and the very old. Food contamination, particularly from unpasteurized cheese, is responsible for many adult cases. Listeria causes purulent leptomeningitis with one important differentiating factor from the other causes of neonatal meningitis: the fetus and the placenta may become infected, with the result being spontaneous abortion or preterm labor. Chronic involvement of the female genital tract leads to habitual abortion. A typical clinical picture in early-onset neonatal Listeria infections includes a maternal flu-like illness, preterm labor with intact membranes, meconium staining, perinatal asphyxia, neonatal respiratory distress, a maculopapulovesicular skin eruption, a monocytic predominance in the endotracheal aspirate, meningitis and intraventricular hemorrhage. CNS involvement is more common in infants with a late onset of infection. Involvement of the meninges and brain consists of miliary granulomas and micro-abscesses. The predilection for purulent meningoencephalitis or involvement of the pons and medulla oblongata in adult Listeria infection is not seen in the fetus and neonate. Hydrocephalus, cystic encephalomalacia, and periventricular cavitation and calcification may be present in survivors2, 4, 34(Fig. 10.8).
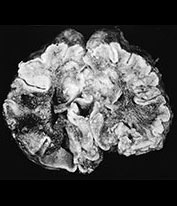
Fig. 10.8 (a-d) Citrobacter diversus - MR in a 5-week-old infant. FLAIR (a), enhanced T1W (b), and T2W (3000/120) (c) axial MR images in a 5-week-old neonatal intensive care unit ëgraduateí demonstrates multiple necrotic and infected cavities with rim enhancement, daughter cysts, and fluid with varying signal intensity in keeping with blood and pus. Pathologic specimen (d) in another patient with Citrobacter demonstrates large white matter abscess.
Fig. 10.8 (e-f) Enterobacter sakazakki. T1 (860/20) weighted sequence in a 34-week premature infant aged 4 days (e), and 6 years (f). The large frontal abscess was clinically silent. At 6 years there is atrophy and gliosis of the right frontal lobe. Development is normal although he suffers from focal convulsions of recent onset. (With permission Dr Mary Rutherford.)
Fig. 10.8 (g-h) Listeria monocytogenes. T1 (860/20) (g) and T2W fast spin echo (3000/208) (h) axial images in a 3-week-old infant born prematurely at 32 weeks following a symptomatic Listeria monocytogenes infection in the mother. There is a large intraventricular hemorrhage with adjacent infarction on the right (arrow). There are extensive periventric cystic lesions on the left (arrowhead). (With permission Dr Mary Rutherford.)
Citrobacter diversus
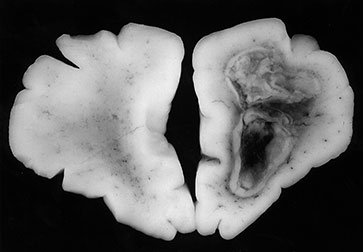
Citrobacter diversus is a Gram-negative enteric bacterium, also with a predilection for the old and the very young. In the debilitated elderly patient, it infects the urinary tract and respiratory system. In the infant, it causes neonatal sepsis and meningitis. Citrobacter sp. rarely infect the infant older than 8 weeks of age, while the premature infant is particularly prone to infection. Poor intracellular survival in the host with an intact, mature immune system is felt to be responsible for the low virulence associated with C. diversus beyond the neonatal period. Acquired vertically or in nursery outbreaks, it is more important as a pathogen for its severity of sequelae than its actual prevalence. Citrobacter accounts for less than 5% of cases of meningitis in the neonate, but leads to brain abscesses in 75% or more of infants with meningitis. Neurological sequelae are present in the majority of the survivors of Citrobacter meningitis. Relapse and recurrent ventriculitis with prolonged persistence in the CNS has been described. Spinal cord abscesses have also been reported. Imaging features are characteristic with multiple, large, ring-enhancing lesions of white matter necrosis, cavitated infarcts and abscess formation. Pathologic features consist of meningeal vascular congestion, ventriculitis with focal ependymal disruption, periventricular and wide-spread white matter necrosis27, 38, 81, 85(Fig. 10.7 and Fig. 10.8).
Fungal meningitis
Fungal meningitis may be acquired intra utero from an ascending chorionic infection, during birth from an infected maternal genital tract, or postnatally. Candida, a saphrophyte of healthy persons, is the most frequent of these fungal agents. Debilitation, immunocompromised state, in-dwelling central lines and antibiotic therapy predispose to candidiasis. Premature infants are particularly susceptible. Systemic candidiasis is reported to occur in 3–5% of very low-birth-weight neonates, with CNS involvement in up to 64% of these. CNS involvement is often difficult to prove antemortem, as concentration within the CSF may be low and the growth rate in culture medium slow. The hallmark of the pathological changes are micro-abscesses. Micro-abscesses may be demonstrated at postmortem in infants with apparently normal MRI. Small macro-abscesses are the most common imaging finding in the very low-birth-weight neonate, with small echogenic rim-like micro-abscesses symmetrically scattered in the subcortical, periventricular and basal ganglia regions. Discrete punctate or rim-enhancing disseminated small macro-abscesses are very well shown on enhanced T1W images. Confluence of macro-abscesses, ventricular dilatation and ventriculitis may also be demonstrated. Differentiation from the lesions of periventricular leukomalacia is based on the pattern and timing of involvement, with the lesions of candidiasis occurring later, and more frequently involving the deep gray structures. Parenchymal involvement is more typical, but ventricular involvement also occurs. Intraventricular strands and debris, thickened irregular choroid plexus, and thickened, irregular ventricular walls are seen. Follow-up imaging demonstrates regression of lesions, although calcified granulomas occur. Invasion of the brain and spinal cord leads to necrotizing encephalitis and encephalomyelitis. Pathologic specimens demonstrate widespread necrotizing encephalitis, granulo-cytic infiltrates, reactive glial proliferation and large abscesses or military micro-abscesses. Acute Candida foci, with infiltrating neutrophils, yeasts and pseudohyphae, may be widespread, involving deep white and gray matter and the subependymal germinal layer. Particular involvement is present in the watershed zones. The smaller chronic foci tend to localize within the cortex, basal ganglia, brain stem nuclei and leptomeninges. In the older infant, Candida tends to cause a purulent leptomeningitis and ventriculitis similar to bacterial agents. Hydrocephalus and CSF loculation are common complications (Fig. 10.9). Aspergillus, extremely rare in the young infant, will cause similar pathological, and therefore imaging, features. Mucormycosis, also extremely rare, leads to infarction due to direct vascular invasion and thrombosis, in addition to the necrosis from parenchymal invasion seen with other fungal infections8, 30, 34, 51, 54, 86.
Fig. 10.9 Candida abscesses in a 15-day-old premature infant. T1 (500/20) (a) and T2W (3000/120) (b) axial images in a premature infant with systemic candidiasis demonstrate multiple foci of small macro-abscesses (arrow). T1 (860/20) (c) and T2 (2700/120) (d) weighted images in a second preterm infant born at 26 weeks and imaged at 41 weeks, 12 weeks after an episode of candidal septicemia. There are multiple lesions within the white matter consistent with calcification of Candida macro-abscesses. Coronal pathologic sections of the cerebellum and brain stem (e) in another patient demonstrate multiple foci of cerebritis.
Infant botulism
Infant botulism is a disease of the slightly older infant, although neonatal cases have been described. Infants under 1 year of age are at risk due to the composition of the intestinal flora. Clostridium botulinum spores ingested with contaminated honey colonize the intestinal tract, bind to intestinal epithelium, germinate, and produce a neurotoxin which is subsequently absorbed. Skeletal muscle is paralyzed from presynaptic blockade of acetylcholine release. Infants present with difficulties eating, intestinal and gastric dilatation, ocular and bulbar paralysis, descending muscle paralysis and apnea. Intracranial manifestations are felt to relate to associated hypoxia. MR features have not been described, although we have seen demyelination of the corpus callosum and enhancing cauda equina nerve roots in a 6-month-old infant who presented with feeding difficulties, ptosis and a history of honey ingestion78.
Tuberculosis
Tuberculosis may occur in the very young infant, but is extremely rare in the neonate. Connatal tuberculosis has been described in the case of active maternal tuberculosis, although it was more common in the pre-antituberculous chemotherapeutic era. Infection occurs via the placenta, aspiration of amniotic fluid during delivery, or following postnatal respiratory exposure. Miliary dissemination is seen, although basal leptomeningitis with brain infarction due to occlusion of the basal perforators is more common (Fig. 10.1). Hydrocephalus, tubercules demonstrating massive caseation and poor peripheral lymphohistiocytic reaction, and late leptomeningeal calcification are complications34, 56.
CONGENITAL INFECTIONS (STORCH)
Considerable progress has been made in preventing or treating congenital infection with immunizations and intrauterine diagnosis and therapy. However, these infections, usually including syphilis, toxoplasmosis, rubella, CMV, HIV and herpes simplex virus (HSV) have not been eliminated. CMV remains the most common, affecting 1 in 100 live births in the US. HIV, Treponema pallidum and Toxoplasma gondii follow with a rising incidence of vertically transmitted HIV. Herpes simplex virus and varicella zoster affect 1 in 5,000, and 1 in 10,000 respectively, while the incidence of rubella has decreased to 1 in 100,000.
Routes of transmission vary somewhat, with HIV and rubella most commonly transmitted in utero, HSV transmitted during birth, and CMV transmitted in utero, intrapartum and postpartum. Additional congenital infections include chicken pox, hepatitis, enterovirus, parvovirus, lymphocytic choriomeningitis virus, Q fever, malaria and tuberculosis31, 43.
Diagnosis of congenital infections, whether they reach the fetus via a hematogenous transplacental route or by ascent through the birth canal, remains challenging. The requisite maternal infections are usually asymptomatic and some infants may be clinically normal at birth, only clinically manifesting occult ocular, audiologic and CNS complications later on. Intrauterine growth retardation, hydrocephalus or microcephaly, echogenic bowel, pseudomeconium ileus, hepatic or brain calcifications, hydrops fetalis, ascites, and pleural or pericardial effusions should suggest intrauterine infection. Specific testing is required. Polymerase chain reaction (PCR) testing on amniotic fluid, for example, is available in suspected cases of Toxoplasmosis gondii infections. Neonatal features may suggest the presence of a congenital infection, although many are not specific. Systemic involvement includes intrauterine growth retardation in rubella, toxoplasmosis and CMV. Hepatosplenomegaly is reported in CMV, rubella, toxoplasmosis, HSV, syphilis, enterovirus and parvovirus B19. Likewise, progressive hearing loss occurs in rubella, CMV, toxoplasmosis and syphilis. Anemia can occur with many of the congenital infections, although it is a particular feature of parvovirus B19 due to the propensity of the virus for the fetal red cell. Other clinical features may be more specific, for example congenital heart disease in rubella, and limb paralysis and cicatrices in varicella. Cutaneous lesions are also useful markers. Petechiae, purpura, jaundice and dermal erythropoieses are seen in toxoplasmosis, rubella and CMV, while symptomatic infants with herpes simplex virus infections may show single or grouped cutaneous vesicles, conjunctivitis and oral ulcers28, 31.
Pathologic and radiologic features differ somewhat amongst the congenital infections, although calcifications and necrosis are hallmarks. Plain films features, such as ‘celery-stalking’ of long bones may occur in syphilis and rubella, while patterns of CNS calcification may also give useful clues. Infants with CMV, for example, frequently have periventricular injury with subsequent necrosis and calcifications. Toxoplasmosis leads to more diffuse calcifications, although basal ganglia and periventricular calcifications are common. Calcifications are also seen in parvovirus and in lymphocytic choriomeningitis virus infections. Branching echogenic foci on brain sonography occurs in the congenital infections, particularly CMV, rubella and HIV but may be seen in a myriad of other disorders such as asphyxia, chromosomal anomalies, fetal alcohol syndrome, and non-immune hydrops. Rubella is associated with vascular lesions and chronic meningoencephalitis, and HSV with necrotic foci and hemorrhage. Rubella and herpes are the infections most likely to have extensive cerebral cortical calcifications, while rubella and CMV lead to micrencephaly. Subependymal cysts occur in CMV and rubella, but also in Zellweger syndrome, following germinal matrix hemorrhage, and D-2-hydroxyglutaric aciduria. Hydrocephalus is common in toxoplasmosis, syphilis and enterovirus. Congenital myxoviruses, including mumps and parainfluenza, also have a predilection for ependymal cells, leading to congenital hydrocephalus14, 31, 52, 75, 79.
< prev |
top |
contents |
next >
SYPHILIS

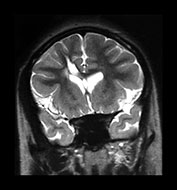
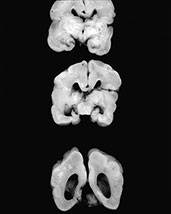
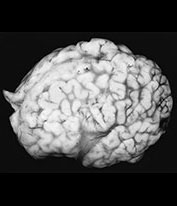
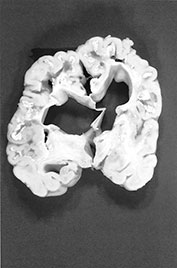
Fig. 10.10 Congenital syphilis. Coronal view of the brain in an adult survivor of congenital syphilis shows focal left cerebral atrophy with deepening of the sulci, superimposed on diffuse brain substance volume loss.
Syphilis infections follow transplacental transmission of treponema usually during or after the 4th month of pregnancy, although infection may be transmitted at any time during pregnancy. Infection of the placenta may lead to stillbirth and premature abortion. The most severely infected fetal survivors are infected relatively late, after week 24 of pregnancy, and there is a high perinatal mortality. The overwhelming majority of infected infants are clinically asymptomatic at birth necessitating careful review of maternal serology and follow-up to avoid long-term CNS complications. Fetal hydrops and hepatomegaly are common. Neonates may demonstrate exanthema, rhinitis and hepatosplenomegaly. ‘Pseudoparalysis’ of the limbs as a result of osteochondritis is a well-known clinical feature in the neonate. Additionally, necrotizing enterocolitis as a result of aortic vascular disease has been reported. There are both meningovascular and parenchymatous forms of neonatal neurosyphilis. Parenchymal disease leads to diffuse cerebral and cerebellar degeneration with microglial proliferation and inflammatory infiltrates. The meningovascular form is associated with perivascular inflammatory exudates, intimal proliferation and leptomeningeal spirochete masses, although parenchymal damage is usually slight. Hydrocephalus may result from syphilitic meningitis. There are occasional superficial cortical infiltrates and necrosis. Latent connatal syphilis (lues tarda) is associated with hearing loss, deformed incisors, saddle nose and tibial deformities. Tabes dorsalis is rare1, 34, 64(Fig. 10.10).
TOXOPLASMA
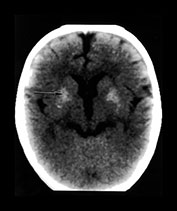
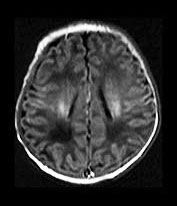
Toxoplasma is a protozoan parasite causing transplacental infection (toxoplasmosis) of the human fetus during or after the 3rd fetal month, and is the third most common fetal brain infection after CMV and HIV. Cats are the terminal host with oocysts excreted in feline feces. Maternal risk factors include exposure to cat excreta during pregnancy, or eating raw, previously unfrozen meat. Contaminated water supply has been linked to one outbreak. The maternal infection is usually asymptomatic, although involvement of the CNS occurs in approximately half of infected fetuses. The rate of infection increases with each trimester, while the actual severity of the infection decreases. Hydrops fetalis, ascites, pleural and pericardial effusions and hydrocephalus may be seen on fetal sonography. While neonates may be symptomatic at birth with clinical features similar to congenital CMV, they are usually asymptomatic. An important differentiating feature would be the head size, as neonates with congenital CMV are commonly microcephalic, while those with congenital toxoplasmosis are more likely to have hydrocephalus. Release of Toxoplasma from cysts leads to an intense inflammatory reaction and granulomatous necrosis. Progressive hydrocephalus occurs in association with turbid, proteinaceous CSF, ependymitis and subsequent aqueductal obstruction. Marked expansion of the atria of the lateral ventricles is common in patients with Toxoplasma-related hydrocephalus. Neuronal migration anomalies are not commonly seen, although delayed myelin maturation is seen on imaging. Basal ganglia and periventricular calcifications are common, may be seen even in asymptomatic children, and may even disappear over time without treatment. Chorioretinitis is extremely common, and may lead to progressive visual loss in otherwise asymptomatic children. Treatment in utero is available and improves outcome; treatment during the first year of life also improves neurological outcome8, 13, 33, 34, 36, 67(Fig. 10.11).
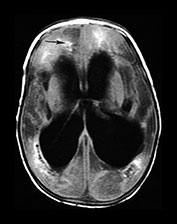
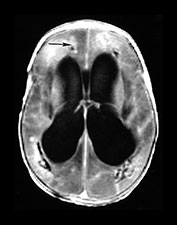
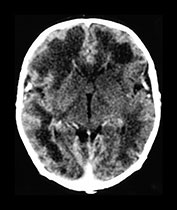
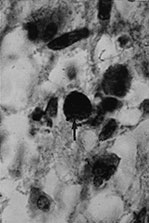
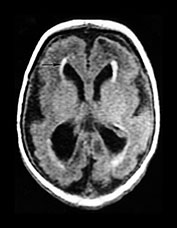
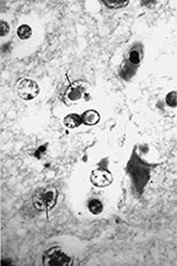
Fig. 10.11 Disappearing calcification in congenital toxoplasmosis. Unenhanced axial images of the brain in a 12-month-old infant shows ventricular enlargement (a). CT at 4 years of age show that brain calcification is lessening over time (b). HE stain, high-power view (c) shows a Toxoplasma cyst (arrow), characteristic of established infection surrounded by inflammatory cells. Cysts may be found around the periphery of necrotic lesions and may survive for years. (CTs of this case are used with permission of Dr Kling Chong of Hospital for Sick Children at Great Ormond Street.)
RUBELLA
Rubella, a single-stranded RNA Togaviridae family virus, causes German measles, a usually self-limited and benign disease in children. Sequelae of congenital rubella, however, particularly acquired during the early part of the first trimester, are severe. The actual incidence of congenital rubella following maternal infection is reported to be low, although during the critical first 12 weeks of pregnancy the fetal infection rate can be as high as 80%. Congenital heart disease is reported in more than half, deafness due to damage to the organ of Corti in approximately half, and visual changes such as cataracts in approximately 40%. Additionally, 40% of survivors have developmental delay. Neurological symptoms are related to viral invasion and replication in brain tissue. Rubella appears to have an antimitotic effect on brain cell multiplication with micrencephaly a common outcome of fetal infection. The main brain tissue cell types infected with in utero rubella virus are the astrocyte and occasionally the neuron. The oligodendrocyte is relatively resistant, leading to a lack of significant demyelination, although delayed myelin maturation occurs.
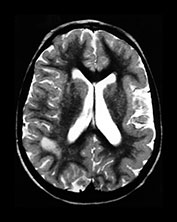
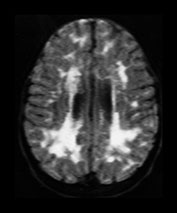
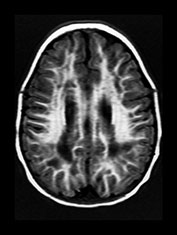
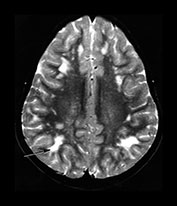
Rubella virus has a particular tendency to involve placental and fetal vascular endothelia. Abnormalities of the cerebral vascular system are present on pathologic specimens in more than half of cases. Focal destruction of the vascular walls, with thickening and proliferation results in luminal narrowing. Mineralizing microangiopathy with arterial occlusion and stroke occur. Outcomes include hydranencephaly, micrencephaly, particular cerebellar atrophy, and calcified brain. ‘Branched candlestick’ appearance of the vessels is seen on brain sonography of neonates. End vessels within the deep white matter and basal ganglia are the most frequently affected in pathologic studies of infants who died of congenital rubella syndrome. This ischemic pattern of involvement is felt to be responsible for the MR changes seen in adult survivors. Psychiatric disturbance in adult survivors has been reported in up to 50% of survivors of symptomatic congenital rubella. Congenital rubella has also been implicated as a significant cause of autism. Linear hyperintense foci in the deep white matter of the frontal and parietal lobes are common, seen in over half of adult survivors with schizophrenia-like symptoms, and in children normal except for deafness. Pediatric survivors may also demonstrate delay in myelin maturation and subcortical and periventricular white matter lesions. In another study of adults with congenital rubella and schizophrenia-like symptoms, small intracranial brain volumes and ventricular enlargement with sparing of the sulci were present4, 8, 16, 34, 35, 37, 46, 53, 60, 61, 74, 79, 80, 87
(Fig. 10.12).
Progressive brain damage with leukoencephalopathy, gliosis and atrophy occurs in a subgroup of infants who develop postnatal progressive rubella panencephalitis. Onset of symptoms, dementia, ataxia and seizures, in this slow virus infection, is between the ages of 8 and 21 years in males with clinical signs of congenital rubella syndrome59. Placental vascular and CNS vascular features in congenital infections with Venezuelan equine encephalitis virus infection are similar to those seen in rubella. Massive cerebral necrosis has been described39.
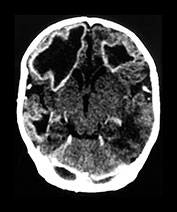
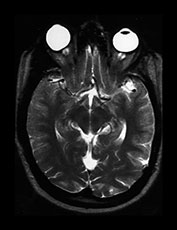
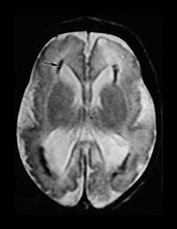
Fig. 10.12 Rubella. Unenhanced axial CT (a) in a 3-day-old with congenital rubella demonstrates punctate calcifications of the basal ganglia (arrow) and low attenuation of the white matter. Ultrasound (not shown) confirmed mineralizing vasculopathy. Axial and coronal MR (b,c) in a teenaged survivor of rubella who is deaf, blind and developmentally delayed demonstrates right frontal white matter and corpus callosum infarct and an abnormal right globe following cataract removal. T1W (860/20) axial image (d) in a term born infant imaged at 5 days. She presented with growth retardation, a petechial rash and thrombocytopenia. Congenital rubella infection was proven on serology. MR imaging showed widespread low S1 within the WM and large focal cystic lesions in the temporal lobes (arrow). (With permission of Dr Mary Rutherford, Hammersmith Hospital.)
CYTOMEGALOVIRUS (CMV)
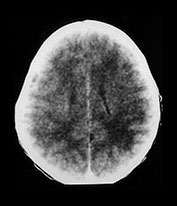
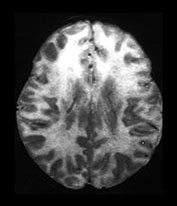
CMV will infect most of us during our lifetimes. CNS complications are uncommon in the otherwise healthy child and adult. CMV poses its greatest risks to the fetus, the premature infant and the immunocompromised infant. The usual route of fetal infection is transplacental occurring during a primary infection of the mother. Thirty to 40% of cases of maternal primary infection will lead to fetal infection. Gestational age at time of infection has little correlation with rate of transmission, or severity of disease expression. Maternal antibodies, which protect the fetus in rubella and toxoplasmosis, do not prevent fetal transmission of the CMV virus, but do lessen the severity of the disease in CMV. Approximately 1% of neonates are born infected with CMV, but the majority will have silent infections following recurrent rather than primary maternal infection. CMV is thought to be the leading infectious cause of sensorineural hearing loss in the postrubella vaccination era, affecting approximately 10% of infected neonates. Mondini malformation with lack of interscalar septum, short and wide internal auditory canal and enlarged vestibular aqueduct are particularly common CT features of CMV survivors with hearing loss7, 35, 50, 77.
A small per cent of infants who have been infected in utero will be symptomatic with jaundice, hepatosplenomegaly, petechiae, microcephaly or chorioretinitis. Half to three-quarters of these infants will have abnormal neuroimaging. Prenatal documentation of atrophy, ventricular enlargement and prominent CSF spaces have been reported. Periventricular calcification and subependymal cysts have been documented on in utero and postnatal cranial imaging. ‘Ring-like’ areas of periventricular lucency have been shown to precede the development of subependymal calcification and are felt to represent foci of subependymal degeneration and inflammation. Subsequent glial scarring and dystrophic calcification occurs. Increased echogenicity of the thalamo-striate arteries has been described on cranial sonography in the presence of congenital CMV, although this feature is not specific for CMV. The presence of an abnormal CT imaging during the neonatal period, with calcification the most common finding, has been shown to correlate well with an adverse neurodevelopmental outcome. Periventricular, and less frequently, basal ganglia, calcifications are reported on CT in 33–43%. This number is lower than that reported during the plain film era, likely due to the increasing identification of less severely affected patients. Periventricular foci of signal abnormality are common on MR, although it is difficult to differentiate calcification from punctate hemorrhage, as calcifications are less well appreciated on MR than on CT
(Figs 10.13–10.15).
Imaging features such as microcephaly and diffuse brain calcifications are shared with Aicardi–Gouthier syndrome, a familial disorder with CSF lymphocytosis
4, 12, 24, 76, 79.
Additionally, several different patterns of damage subsequent to congenital CMV infection have been described on MRI and reflect features well described on pathologic specimens. Lissencephaly with a thinned cerebral cortex, enlarged lateral ventricles, diminished white matter volume, delay in myelination and small cerebellum support an insult to the germinal zone and therefore infection prior to 16–18 weeks of gestation. Localized dysplastic cortex (polymicrogyria) with thickened irregular cortices and diminished white matter is felt to indicate infection late in the migrational phase or in the organizational phase between 18 and 24 weeks. Focal polygyria in patients in this population is most commonly identified within the frontal lobes, and to a lesser degree within the temporal lobes. A normal gyral pattern with abnormal white matter signal supports infection in the third trimester, but it is not always possible to predict the pattern of brain abnormality from the reported timing of maternal infection4,6,47 (Fig. 10.15).
Fig. 10.13 Mineralizing microangiopathy and periventricular cysts in CMV. Sonography (a) in a neonate with documented CMV infection demonstrates the ëcandelabraí configuration of mineralizing microangiopathy. Subependymal cysts (arrow) (b) are seen in a second neonate. Unenhanced CT (c) demonstrates typical calcifications as well as cerebellar hypoplasia (arrow), a common feature. Adjacent cut (d) from the same patient shows additional intraventricular hemorrhage (arrow). HE stain, low-power view (e) in pathologic specimen of CMV infection demonstrates intraparenchymal and perivascular (thin arrow) inflammatory infiltrates, as well as several cytomegalic cells (thick arrow) with intranuclear inclusions.
Fig. 10.14 Chronic CMV infection in term neonate. T1 (450/12) (a) and T2 (3000/80) (b) weighted images reveal arrested brain development with primitive Sylvian fissures and a smooth cortical surface in a full-term infant with microcephaly and documented congenital CMV infection. Note the periventricular calcifications (arrows). Coronal pathologic sections of brain (c) in another patient reveal periventricular and white matter calcifications (chalky white areas) in a case of congenital CMV infection and arrested brain development.
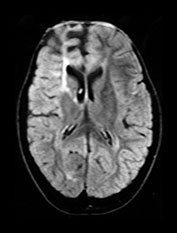
Fig. 10.15 Maldevelopment of brain in a 3-year-old following congenital CMV. T2 (2800/90) (a) axial image reveals a small left cerebral hemisphere, extensive white matter demyelination and destruction, and frontal lobes carpeted with polygyria in a 3-year-old with a known intrauterine infection occurring during the 8th fetal month. Abnormal white matter in CMV. T1 (860/20) (b) and T2 (2700/120) (c) weighted images in a 2-year-old infant with congenital CMV with maternal flu-like illness in the first trimester. There is widespread abnormal signal intensity within the white matter, this is most marked in the T2 weighted image. Her development at 7 years is within normal limits. (With permission of Dr Mary Rutherford, Hammersmith Hospital.)
CONGENITAL HIV
Congenital HIV is now the second most frequent viral infection of the newborn43. Usual transmission is in utero, although mother-to-child transmission may also occur postpartum via breast milk. Invasion of the CNS by HIV occurs at the time of the initial infection, prior to the period of symptomatic AIDS. Initially specific immune responses of neutralizing antibodies and cytotoxic T-lymphocytes inhibit viral replication. The virus, however, incites a subacute encephalitis with inflammatory T-cell reaction with perivascular mononuclear inflammatory cell infiltrates, leptomeningitis, and immune activation of brain tissue with increasing microglial cells and cytokine production. There is damage to the oligodendrocytes by cytokines and myelin pallor. Late neuronal loss occurs. Gliosis and brain atrophy are early features of adult infections, while impaired brain growth is characteristic of congenital infections. Multinucleated giant cell encephalitis and vacuolar myelopathy are less common in pediatric AIDS than in adults, although corticospinal tract degeneration does occur41, 43.
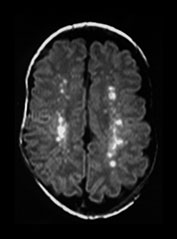
Early clinical signs of HIV-related illness in the very young infant include failure to thrive, hepatosplenomegaly, pulmonary lymphoid hyperplasia, chronic diarrhea, thrush and recurrent bacterial infections. The most common sign of early HIV-related CNS encephalopathy is delay in acquisition of psychomotor milestones. Later on there may be loss of milestones, acquired microcephaly, and bilateral corticospinal tract involvement. Frequently, however, CNS symptomatology is minor in the first decade, with CNS disease ranging from 8% in asymptomatic children to 60% in children with advanced disease. CNS symptoms include a static–stable encephalopathy or a subacute slowly progressive course. Progressive mineralizing vasculopathy of the basal ganglia, frequently present at birth, is the most common abnormal finding on neuropathology and imaging (Fig. 10.16). This calcification is shown on pathologic specimens to be in areas of intimal proliferation. Vascular striations are seen on brain sonography, and diffuse hazy increased density of the basal ganglia is often seen on CT. While cerebrovascular disease has been documented in one study to be present at autopsy in 24% of children with HIV, stroke occurs in 1–2%, less commonly in the older child than the infected adult and least commonly in the infant. Aneurysmal dilatation of the vessels of the circle of Willis occurs and has been reported as early as 6 months of age11, 71.
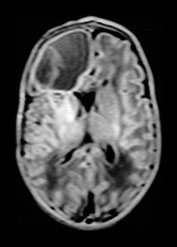
Fig. 10.16 Mineralizing microangiopathy and white matter lesions in congenital HIV. Axial CT (a) in a 2-month-old infant with known vertical transmission of HIV demonstrates hazy mineralizing micro-angiopathy of the basal ganglia and thalami (arrow). Axial T2W MR (2800/90) (b) in a 6-year-old child with vertically acquired HIV and failure to thrive shows focally abnormal signal within the subcortical white matter (arrow). This child subsequently succumbed to the sequelae of massive aneurysmal dilatation of the vessels of the circle of Willis.
Routine MRI in congenital HIV is initially normal and despite the identification of HIV within fetal brain tissue as early as 15 weeks’ gestation, there are no associated brain malformations. Delay in myelin maturation is common in the infected young infant. Atrophy, HIV white matter disease particularly involving the cortical white matter, and progressive multifocal leukoencephalopathy (PML) are late findings in the child with vertically acquired disease, as is symptomatic brain involvement by opportunistic infections such as toxoplasmosis and CMV. MR spectroscopy may be useful even when the routine MRI is normal. Proton MR spectroscopy of the brain is abnormal in neonates exposed to HIV in utero, although this does not differentiate infected children from uninfected children. A non-specific amino acid peak in the 2.1–2.6 ppm area overlapping the N-acetylaspartic acid peak, high N-acetyl aspartate (NAA)-to-creatine and high choline-to-creatine ratios were felt to possibly result from the indirect effects of HIV, such as intrauterine growth retardation. Elevated choline is postulated to result from either myelin breakdown or from microglial and astrocytic hypertrophy. The decrease in the NAA-to-creatine ratio seen in older children and adults with symptomatic, progressive HIV encephalopathy was not seen in the HIV-exposed neonates. Loss of NAA in progressive HIV encephalopathy is likely secondary to loss of cortical neurons, as well as to loss of NAA within the axonal processes. Lactate has also been described, possible secondary to the glycolytic flux of the perivascular macrophages known to be present in symptomatic patients. The decreased NAA-to-creatine ratio and the lactate peak demonstrated in children with progressive HIV encephalopathy have been shown to improve following antiretroviral therapy5, 9, 19, 62, 68.
HERPES SIMPLES VIRUS (HSV)
HSV, most commonly transmitted during birth, may cause significant damage to the developing brain whether acquired in utero or perinatally. HSV-2 infections account for 80–90% of all neonatal and almost all of the congenital herpes virus cases. Endothelial cell infection with swelling and small vessel necrosis leads to early fetal brain destruction and fetal loss. Twenty per cent of infants with neonatal HSV present with isolated CNS involvement, typically during the second or third week of life. These infants may have non-specific clinical signs such as irritability, high-pitched cry, fever or poor feeding. Mortality is high if the CNS is involved in disseminated neonatal HSV. A smaller group, infected in utero, will present with microcephaly, cataracts and intrauterine growth retardation. Herpetic skin rashes are not universally seen and the maternal infection may be unsuspected, as primary infections, which are the highest risk to the fetus, are characteristically asymptomatic.
Brain involvement in neonatal encephalitis is diffuse, bilateral and typically does not spare the white matter. Predisposition for the temporal lobes does not occur. Sequential imaging typically demonstrates progressive brain edema with subsequent encephalomalacia and occasional cyst formation although unenhanced imaging early in the disease may be normal. Necrosis of brain substance may be severe and there is a predilection to involve the cerebellum, basal ganglia and brain stem. Increased cortical attenuation has been described in neonatal cases, possibly due to cortical vasodilatation rather than petechial hemorrhage or microcalcification. Hemorrhage or calcific densities are present in the thalamus, basal ganglia, peri-insular cortex, periventricular white matter and at the gray–white junction in infants. Progressive, diffuse brain calcification may rival only rubella in the degree of calcification. Mineralized depositions confined to the mitochondria have been reported in disintegrated cells which contain herpes simplex virions. Relapse or persistence of infection is known despite appropriate antiviral therapy, particularly in association with frequent cutaneous relapses.
The pattern of destruction in infants, different from that in older children and adults, is likely due not only to the response of the developing brain to herpes encephalitis, but to the difference in acquisition of the infectious agent. HSV-1, typically, although not exclusively an infection of the older child and adult, usually enters the intracranial compartment via the olfactory nerve leading to specific involvement of the inferior and middle temporal gyri in older patients. Intrauterine acquired HSV-1 infection may also be associated with severe and progressive atrophy and calcification. Neuronal migration disorders have been reported in association with fetally acquired HSV-14, 8, 10, 17, 18, 26, 34, 42, 49, 55, 58, 65, 77(Fig. 10.17).
Fig. 10.17 (a-i) Herpes simplex. Unenhanced axial CT in a 2-month-old infant (a) shows mild brain edema in a child presenting with fever, seizures and encephalopathy. T2W MR (b) shows more pronounced edema with high signal intensity most marked in the frontal white matter. Subacute sonogram demonstrates multifocal cystic lesions (c), while follow-up unenhanced CT (d) shows severe multicystic encephalomalacia with extensive cortical calcifications. Extensive cerebral necrosis and encephalomalacia (e) is present in a case of HSV-2 encephalitis. Autopsy specimen in an infant with HSV-1 encephalitis (f), shows evident cerebral atrophy with deep sulci, while the cut surface of the brain (g) demonstrates extensive calcification (chalky white material) and ventriculomegaly. H&E stain, medium-power view
(h) demonstrates area of necrosis surrounded by reactive gliosis. H&E: high-power view (i) demonstrates intranuclear viral inclusions (arrows) in a different infant with HSV-1 encephalitis.
Fig. 10.17 (j-l) Neonatal herpes simplex encephalitis. T1W MR (860/20) at 5 days (j), 12 days (k) and 3 months (l) in an infant with seizures on day 3 of life. The initial images show minimal low signal intensity within the white matter normal in appearance but 1 week later there is extensive hemorrhagic infarction of WM and cortex (k) leading to atrophy (l).
CONGENITAL VARICELLA ZOSTER
Congenital varicella zoster virus infections are less common than the other congenital infections. Maternal immunity is high, and infectivity of the fetus is apparently low. The congenital varicella syndrome typically follows 2–4% of maternal infection occurring prior to 20 weeks’ gestation. A specific feature is cicatrix (lightning-flash skin lesions) in a dermatomal distribution. Limb hypoplasia and weakness follow intrauterine damage to the cervical or lumbosacral plexi; segmental spinal cord necrosis, intrauterine growth restriction (IUGR), cataracts, chorioretinitis and microphthalmia occur. Intracranial findings are the sequelae of necrotizing encephalitis and include hydrocephalus, porencephaly, hydranencephaly, calcifications, and malformations associated with intracranial vascular compromise, such as polymicrogyria or focal lissencephaly. Severe microcephaly may be an isolated feature and cerebellar hypoplasia is reported. Zoster may be the clinical presentation in infants infected after 20 weeks’ gestation. Congenital varicella acquired during peripartum maternal infection leads to a presentation which resembles varicella in the immunocompromised host with pneumonia, progressive hepatic failure with clotting abnormalities and hemorrhage, and a high mortality rate (Fig. 10.18). Other infectious causes of disseminated intra-vascular coagulopathy in the neonate include enterovirus family, herpes simplex and, occasionally, toxoplasmosis4, 23, 44, 66, 72.
Fig. 10.18 Varicella. Fulminant varicella infection in an autopsy specimen of a 6-month-old infant with disseminated intravascular coagulation and extensive petechiae. (a) Term born infant with a maternal history of chickenpox at 15 weeks’ gestation. He presented with scoliosis, cryptorchidism, micropthalmia and hypotonia. T1W (860/20) image at 8 days of age. (b) There is extensive cortical and WMinfarction (arrow) in an appropriately mature brain. There were no congenital malformations within the brain. (With permission of Dr Mary Rutherford, Hammersmith Hospital.)
< prev |
top |
contents |
next >
- Neonatal brain infections may be acquired in utero, intrapartum or postnatally.
- Sequelae depend upon the stage of brain maturation at the time of infection, the status of the developing immune system, and the infectivity, dose and actual target cell of the infecting agent.
- Standard imaging protocols with contrast enhancement should be used with suspected infections.
- Computed tomography may be necessary to confirm the presence and the extent of calcification.
- Different patterns of pathologic damage in the developing brain lead to imaging appearances different from those in the fully formed brain; early infections affect organogenesis and later infections often lead to brain destruction.
- Despite the variability in patterns of damage, imaging differentiation amongst the infectious agents may be possible.
< prev |
top |
contents |
next >
- Aicardi J (1992) Diseases of the Nervous System in Childhood. Oxford, MacKeith Press.
- Alliet P, Van Lierde S, Bruylants B et al. (1992) [Neonatal listeriosis]. Tijdschr Kindergeneeskd 60(1), 18–21.
- Arvin B, Neville LF, Barone FC et al. (1996) The role of inflammation and cytokines in brain injury. Neurosci Biobehav Rev 20(3), 445–452.
- Bale JF and Murph JR (1997) Infections of the central nervous system in the newborn. Clinics in Perinatology 24(4), 787–806.
- Barker PB, Lee RR and McArthur JC (1995) AIDS dementia complex: evaluation with proton MR spectroscopic imaging. Radiology 195, 58–64.
- Barkovich AJ and Lindan CE (1994) Congenital cytomegalovirus infection of the brain: imaging analysis and embryologic considerations. AJNR 15(4), 703–715.
- Bauman NM, Kirby-Keyser LJ, Dolan KD et al. (1994) Mondini dysplasia and congenital cytomegalovirus infection. J Pediatr 124(1), 71–78.
- Becker LE (1992) Infections of the developing brain. AJNR 13, 537–549.
- Belman AL (1990) Aids and pediatric neurology. Neurologic Clinics 8(3), 571–603.
- Benator RM, Magill HL, Gerald B et al. (1985) Herpes simplex encephalitis: CT findings in the neonate and young infant. AJNR 6(4), 539–543.
- Bode H and Rudin C (1995) Calcifying arteriopathy in the basal ganglia in human immunodeficiency virus infection. Pediatr Radiol 25, 72–73.
- Boppana SB, Fowler KB, Vaid Y et al. (1997) Neuroradiographic findings in the newborn period and long-term outcome in children with symptomatic congenital cytomegalovirus infection. Pediatrics 99(3), 409–414.
- Bowie WR, King AS, Werker et al. (1997) Outbreak of toxoplasmosis associated with municipal drinking water. Lancet 350, 173–177.
- Chang YC, Huang CC and Liu CC (1996) Frequency of linear hyperechogenicity over the basal ganglia in young infants with congenital rubella syndrome. Clin Infect Dis 22(3), 569–571.
- Chang YC, Huang CC, Wang ST et al. (1997) Risk factor of complications requiring neurosurgical intervention in infants with bacterial meningitis. Pediatr Neurol 17(2), 144–149.
- Chantler JK, Smyrnis L and Tai G (1995) Selective infection of astrocytes in human glial cell cultures by rubella virus. Lab Invest 72(3), 334–340.
- Cleveland RH, Herman TE, Oot RF et al. (1987) The evolution of neonatal herpes encephalitis as demonstrated by cranial ultrasound with CT correlation. Am J Perinatol 4(3), 215–219.
- Corey L, Whitley RJ, Stone EF et al. (1988) Difference between herpes simplex virus type 1 and type 2 neonatal encephalitis in neurological outcome. Lancet 1(8575–6), 1–4.
- Cortey A, Jarvik JG, Lenkinski RE et al. (1994) Proton MR spectroscopy of brain abnormalities in neonates born to HIV-positive mothers. AJNR 15, 1853–1859.
- Dambska M and Laure-Kamionowska M (1998) The morphological picture of developing meningo-encephalitis in central nervous system. Folia Neuropathol 36(4), 205–210.
- Dammann O and Leviton A (1998) Infection remote from the brain, neonatal white matter damage, and cerebral palsy in the preterm infant. Semin Pediatr Neurol 5(3), 190–201.
- Daneman A, Lobo E and Mosskin M (1998) Periventricular band of increased echogenicity: edema or calcification? Pediatr Radiol 28, 83–85.
- Deasy NP, Jarosz JM, Cox TC et al. (1999) Congenital varicella syndrome: cranial MRI in a long-term survivor. Neuroradiology 41, 205–207.
- Dias JMJ, van Rijckevorsel GH, Landriue P et al. (1984) Prenatal cytolomegalovirus infection and cerebral microgyria: evidence for perfusion failure, not disturbance of histogenesis, as the major cause of fetal cytomegalovirus encephalopathy. Neuropediatrics 15, 18–24.
- Egelhof JC (1997) Infections of the central nervous system. In: Ball WS (Ed.) Pediatric Neuroradiology. Philadelphia, Lippincott-Raven, pp. 273–318.
- Enzmann D, Chang Y and Augustyn G (1990) MR findings in neonatal herpes simplex encephalitis type 2. J Comput Assist Tomogr 14(3), 453–457.
- Eppes SC, Woods CR, Mayer AS et al. (1993) Recurring ventriculitis due to Citrobacter diversus: clinical and bacteriologic analysis. Clin Infect Dis 17(3), 437–440.
- Epps RE, Pittelkow MR and Su WP (1995) TORCH syndrome. Semin Dermatol 14(2), 179–186.
- Ersahin Y, Mutluer S and Guzelbag E (1994) Brain abscess in infants and children. Childs Nerv Syst 10(3), 185–189.
- Felderhoff-Mueser U, Rutherford M, Squier W et al. (1999) Relation between magnetic resonance images and histopathological findings of the brain in extremely sick preterm infants. Am J Neuroradiol 20, 1349–1357.
- Ford-Jones EL (1999) An approach to the diagnosis of congenital infections. Paediatr Child Health 4(2), 109–112.
- Ford-Jones EL and Ryan G (1999) Implications for the fetus of maternal infections in pregnancy. In: Armstrong A and Cohen J (Eds) Infectious Diseases. London, Mosby, pp. 55.1–55.14.
- Foulon W, Villena I, Stray-Pedersen B et al. (1999) Treatment of toxoplasmosis during pregnancy: a multicenter study of impact on fetal transmission and children’s sequelae at age 1 year. Am J Obstet Gynecol 180(2), 410–415.
- Friede RL (1989) Developmental Neuropathology, 2nd edn. Berlin, Springer.
- Friedman S and Ford-Jones EL (1999) Congenital cytomegalovirus infection – an update. Paediatr Child Health 4(1), 35–38.
- Friedman S, Ford-Jones LE, Toi A et al. Congenital toxoplasmosis: prenatal diagnosis, treatment and postnatal outcome. Prenat Diagn 19, 330–333.
- Frey TK (1997) Neurological aspects of rubella virus infection. Intervirology 40(2–3), 167–175.
- Gallagher PG and Ball WS (1991) Cerebral infarctions due toCNS infection with Enterobacter sakazakii. Pediatr Radiol 21(2), 135–136.
- Garcia-Tamayo J (1992) Teratogenic effect of the Venezuelan equine encephalitis virus: a review of the problem. Invest Clin 33(2), 81–86.
- Givner LB and Kaplan SL (1993) Meningitis due to Staphylococcus aureus in children. Clin Infect Dis 16(6), 766–771.
- Gray F, Scaravilli F, Everall I et al. (1996) Neuropathology of early HIV-1 infection. Brain Pathol 6(1), 1–15.
- Gray PH, Tudehope DI and Masel J (1992) Cystic encephalomalacia and intrauterine herpes simplex virus infection. Pediatr Radiol 22(7), 529–532.
- Griffith BP and Booss J (1994) Neurologic infections of the fetus and newborn. Neurol Clin 12(3), 541–564.
- Grose C (1994) Congenital infections caused by varicella zoster virus and herpes simplex virus. Semin Pediatr Neurol 1(1), 43–49.
- Haimes AB, Zimmerman RD, Morgello S et al. (1989) MR imaging of brain abscesses. AJR 152, 1073–1085.
- Harwood-Nash DC, Reilly BJ and Turnbull I (1970) Massive calcification of the brain in a newborn infant. AJR 58(3), 528–532.
- Hayward JC, Titelbaum DS, Clancy RR et al. (1991) Lissencephaly–pachygyria associated with congenital cytomegalovirus infection. J Child Neurol 6(2), 109–114.
- Hazebroek FW, Tibboel D, Leendertse-Verloop K et al. (1991) Evaluation of mortality in surgical neonates over a 10-year period: nonpreventable, permissible, and preventable death. J Pediatr Surg 26(9), 1058–1063.
- Herman TE, Cleveland RH, Kushner DC et al. (1985) CT of neonatal herpes encephalitis. AJNR 6(5), 773–775.
- Hicks T, Fowler K, Richardson M et al. (1993) Congenital cytomegalovirus infection and neonatal auditory screening. J Pediatr 123(5), 779–782.
- Huang CC, Chen CY, Yang HB et al. (1998) CNS candidiasis in very low-birth-weight premature neonates and infants: US characteristics and histopathologic and MR imaging correlates in 5 patients. Radiology 209, 49–56.
- Hughes P, Weinberger E and Shaw DW (1991) Linear areas of echogenicity in the thalami and basal ganglia of neonates: an expanded association. Radiology, 179(1), 103–105.
- Hwa HL, Shyu MK, Lee CN et al. (1994) Prenatal diagnosis of congenital rubella infection from maternal rubella in Taiwan. Obstet Gynecol 84(3), 415–419.
- Incesu L, Akan H and Arslan A (1994) Neonatal cerebral candidiasis: CT findings and clinical correlation. J Belge Radiol 77(6), 278–279.
- Jay V, Becker LE, Blaser S et al. (1995) Pathology of chronic herpes infection associated with seizure disorder: a report of two cases with tissue detection of herpes simplex virus 1 by the polymerase chain reaction. Pediatr Pathol Lab Med 15, 131–146.
- Kang GH and Chi JH (1990) Congenital tuberculosis – Report of an autopsy case. J Korean Med Sci 5(1), 59–64.
- Kaufman D, Kilpatrick L, Hudson RG et al. (1999) Decreased superoxide production, degranulation, tumor necrosis factor, alpha secretion and CD11b/CD18 receptor expression by adherent monocytes from preterm infants. Clin Diagn Lab Immunol 6(4), 525–529.
- Kubota T, Kusaka H, Hirano A et al. (1985) Ultrastructural study of early stage of calcification in herpes simplex encephalitis. Acta Neuropathol 68(1), 77–79.
- Kuroda Y and Matsui M (1997) [Progressive rubella panencephalitis]. Nippon Rinsho 55(4), 922–925.
- Lane B, Sullivan EV, Lim KO et al. (1996) White matter MR hyperintensities in adult patients with congenital rubella. AJNR 17, 99–103.
- Lim KO, Beal DM, Harvey RL et al. (1995) Brain dysmorphology in adults with congenital rubella plus schizophrenia like symptoms. Biol Psychiatry 37(11), 764–776.
- Lu D, Pavlakis SG, Frank Y et al. (1996) Proton MR spectroscopy of the basal ganglia in healthy children and children with AIDS. Radiology, 199, 423–428.
- Mathews VP, Kuharik MA, Edwards MK et al. (1989) Gd-DTPA-enhanced MR imaging of experimental bacterial meningitis: evaluation and comparison with CT. AJR 152, 131–136.
- Nathan L, Twickler DM, Peters MT et al. (1993) Fetal syphilis: correlation of sonographic findings and rabbit infectivity testing of amniotic fluid. J Ultrasound Med 12(2), 97–101.
- Noorbehesht B, Enzmann DR, Sullender et al. (1987) Neonatal herpes simplex encephalitis: correlation of clinical and CT findings. Radiology 162(3), 813–819.
- Ong CL and Daniel ML (1998) Antenatal diagnosis of a porencephalic cyst in congenital varicella-zoster virus infection. Pediatr Radiol 28, 94.
- Patel DV, Holfels EM, Vogel NP et al. (1996) Resolution of intracranial calcifications in infants with treated congenital toxoplasmosis. Radiology, 199(2), 433–440.
- Pavlakis SG, Lu D, Frank Y et al. (1998) Brain lactate and N-acetylaspartate in pediatric AIDS encephalopathy AJNR 19, 383–385.
- Puvabanditsin S, Wojdylo E, Garrow E et al. Group B streptococcal meningitis: a case of transverse myelitis with spinal cord and posterior fossa cysts. Pediatr Radiol 27, 317–318.
- Sage MR and Wilson AJ (1994) The blood–brain barrier: an important concept in neuroimaging. AJNR 15, 601–622.
- Shah SS, Zimmerman RA, Rorke LB et al. (1996) Cerebrovascular complications of HIV in children. AJNR 17, 1913–1917.
- Sheffer IE, Baraitser M and Brett EM (1991) Severe microcephaly associated with congenital varicella infection. Dev Med Child Neurol 33(1), 916–920.
- Stamos JK and Rowley AH (1994) Timely diagnosis of congenital infections. Pediatr Clin North Am 41(5), 1017–1033.
- Sugita K, Ando M, Makino M et al. (1991) Magnetic resonance imaging of the brain in congenital rubella virus and cytomegalovirus infections. Neuroradiology 33(3), 239–242.
- Takano T (1994) [Pathogenesis of congenital hydrocephalus: role of transplacental myxovirus infection]. No To Hattatsu 26(3), 206–210.
- Tassin GB, Maklad NF, Stewart RR et al. (1991) Cytomegalic inclusion disease: intrauterine sonographic diagnosis using findings involving the brain. AJNR 12, 117–122.
- Tien RD, Felsberg GJ and Osumi AK (1993) Herpesvirus infections of the CNS: MR findings. AJR 161, 167–176.
- Tollofsrud PA, Kvittingen EA, Granum PE et al. (1998) [Botulism in newborn infants]. Tidsskr Nor Laegeforen 20, 118(28), 4355–4356.
- Toma P, Magnano GM, Mezzano P et al. (1989) Cerebral ultrasound images in prenatal cytomegalovirus infection. Neuroradiology 31, 278–279.
- Trottier G, Srivastava L and Walker CD (1999) Etiology of infantile autism: a review of recent advances in genetic and neurobiological research. J Psychiatry Neurosci 24(2), 103–115.
- Tse G, Silver M, Whyte H et al. (1997) Neonatal meningitis and multiple brain abscesses due to Citrobacter diversus. Pediatr Pathol Lab Med 17(6), 977–982.
- Weingarten K, Zimmerman RD, Becker LE et al. (1989) Subdural and epidural empyemas: MR imaging. AJR 152, 615–621.
- Weller RO, Engelhardt B and Phillips MJ (1996) Lymphocyte targeting of the central nervous system: a review of afferent and efferent CNS-immune pathways. Brain Pathol 6(3), 275–288.
- Wong TT, Lee LS, Wang HS et al. (1989) Brain abscesses in children – a cooperative study of 83 cases. Child’s Nerv Syst 5, 19–24.
- Woods CR, Mason EO and Kaplan SL (1992) Interaction of Citrobacter diversus strains with HEP-2 epithelial and human umbilical vein endothelial cells. J Infect Dis 166, 1035–1044.
- Yamaguchi K and Goto N (1993) An autopsy case of brain candidiasis in premature infant: morphology and intraparenchymal distribution of Candida foci. No To Hattatsu 25(4), 369–373.
- Yoshimura M, Tohyama J, Maegaki Y et al. (1996)[Computed tomography and magnetic resonance imagingof the brain in congenital rubella syndrome]. No To Hattatsu 28(5), 385–390.
Visit the author's website at
www.maryrutherfordimaging.co.uk
< prev |
top |
contents |
next >